Dispersion vs Diffusion – Taylor Dispersion
2025-07-19
Dispersion and diffusion are commonly misunderstood. Both refer to a “spreading” phenomenon but have different mechanisms. Diffusion results from the random motion of molecules and is characterised by a diffusion coefficient, . Dispersion on the other hand results from the variable flow-path of a fluid.
To illustrate, let’s look at Taylor dispersion for steady laminar flow in a long pipe with a Poiseuille flow radial velocity distribution:
Giving rise to the following shape:
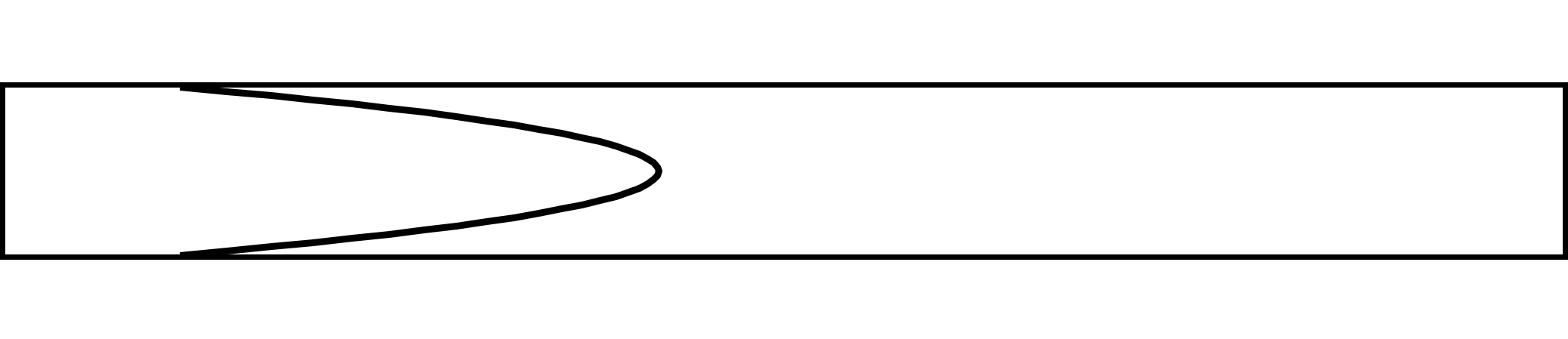
Suppose we fill the pipe with species A between two planes at a known concentration. Taylor assumed that the axial molecular diffusion is negligible compared to the radial molecular diffusion. After time there will be two concentration fronts:
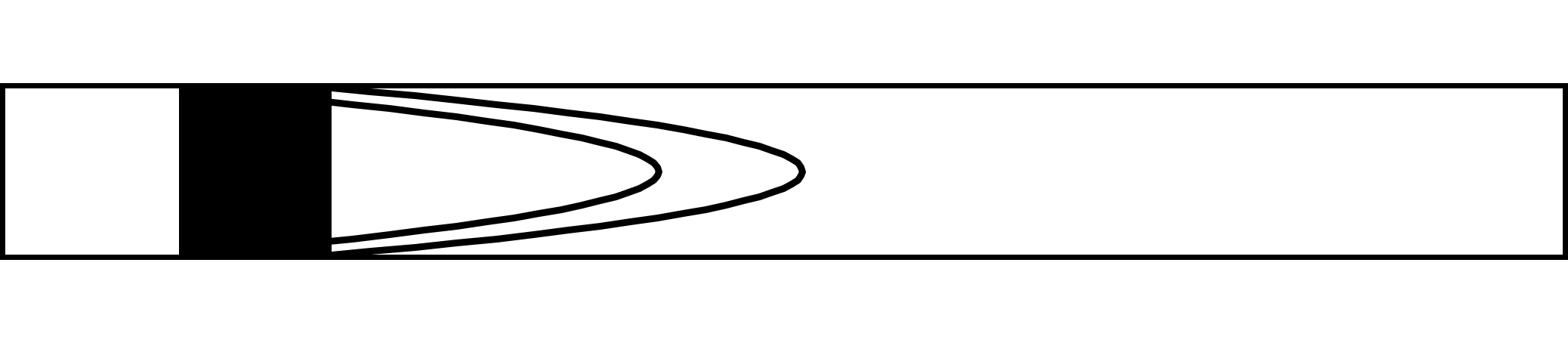
Rapid spreading of the tracer in the radial direction results in a profile like so:
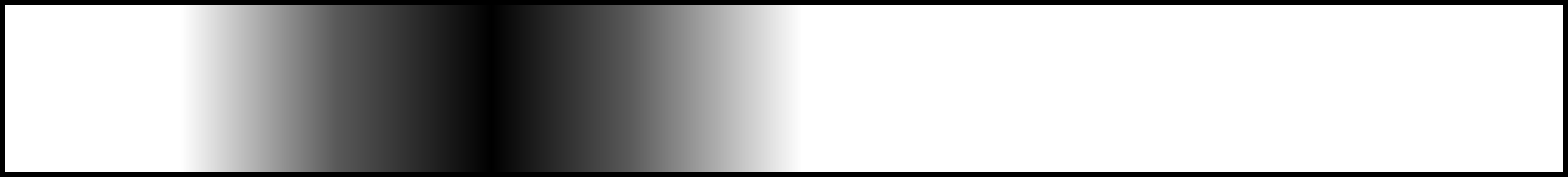
Therefore, the dispersed concentration profile is caused by the velocity profile, and not due to axial molecular diffusion.
The axial dispersion coefficient falls out of this analysis as:
Importantly, is not a property of the fluid and depends on the pipe radius and mean velocity. In fact, is inversely proportional to the diffusion coefficient!
Accounting for axial molecular diffusion Aris modified the equation for Taylors axial dispersion coefficient to be:
Taylor dispersion is ubiquitous in chemical engineering and important for gas separation using structured adsorbents – a future topic.