Joule-Thomson Effect
2025-07-24
Say we have a pressure cylinder of gas at 150 barg and we crack the valve open, will the temperature of the expanded gas increase or decrease? Well, it all depends on the gas in question and the temperature of that gas in the bottle.
Most “real” gases will cool during expansion, but at higher temperatures, this effect is reversed, and throttling will warm the gas. This phenomenon is known as the Joule-Tompson effect, and it is a feature of “real” gases as opposed to ideal gases, where there is no JT effect.
To quantify the magnitude and direction of warming/cooling, we define the JT coefficient as:
Plotting the JT coefficient for helium, hydrogen, nitrogen, argon and carbon dioxide, we can see the inversion temperature of each gas:
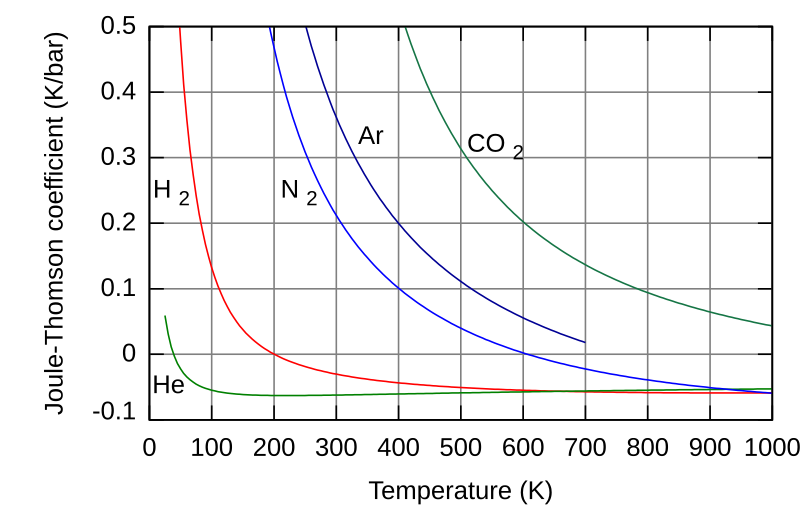
The inversion temperature is defined when the JT coefficient is zero.
For hydrogen at ambient pressure, the inversion temperature is ca. 202 K (-71 degC). This has implications for hydrogen liquefaction cycles, as the initial cool down would be impossible with a simple JT valve. Instead, hydrogen would need to be pre-cooled below its inversion temperature before this could be an effective strategy for hydrogen liquefaction. The same practical problems exist for helium as well.